


Curious about what Stoichiometry means? Don't you worry because I am here to clarify all of your questions. Let's go!

Stoichiometry is derived from the Greek words “stoicheion” which means elements and “metron” which means measure. Known as the Mathematics of Chemical Formulas, stoichiometry is the study of the quantitative relationships that can be derived from chemical formulas and from chemical equations. It is also the precise measurement of the reactants to know the products in a reaction. It encompasses the relationship of quantities (mass of substance or volume of gas) in a chemical change according to the balanced chemical equation.
All reactions are dependent on how much stuff you have. Stoichiometry helps you figure out how much of a compound you will need, or maybe how much you started with. We can say that reactions depend on the compounds involved and how much of each compound is needed.
When doing stoichiometric problems, we have to observe and deal with the following factors:
- Mass of Reactants (chemicals before the reaction)
- Mass of Products (chemicals after the reaction)
- Chemical Equations
- Molecular Weights of Reactants and Products
- Formulas of Various Compounds


Reactant – one of the starting substances involved in a chemical reaction.
Product – a new substance formed during chemical reaction.

Mole - A mole is the amount of pure substance containing the same number of chemical units as there are atoms in exactly 12 grams of carbon-12 (i.e., 6.023 X 1023). The mass in grams of one mole of a compound is equal to the molecular weight of the compound in atomic mass units. One mole of a compound contains 6.02 x 10^23 molecules of the compound. The mass of 1 mole of a compound is called its molar weight or molar mass.
STOICHIOMETRY CONCERNING MOLE-MOLE PROBLEMS
(a type of calculation that relates the moles of two substances
participating in a balanced chemical equation.)

Use the ratio and proportion method to solve this problem.

Cross multiply and divide afterwards to find the unknown value.
-
You can also use the dimensional analysis in this problem:

(b)
Apply the same steps on the next problem.

-
You can also use the dimensional analysis in this problem:
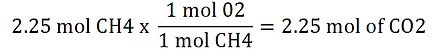

Apply the same steps used in the previous problem, however, multiply 6.02 x 1023 to the denominator of the right hand side of the equation because the number of particles is what we are looking for.

-
You can also use the dimensional analysis in this problem:




-
You can also use the dimensional analysis in this problem:

